How to Choose a Quick Turn PCBA Prototyping Service for Medical Devices
When you select a quick turn pcba prototype medical device service, you need to focus on what matters most for medical devices: reliability, compliance, and speed. Medical device prototyping presents unique challenges that demand special attention. You must optimize for low power consumption, miniaturization, and durability. Manufacturers must meet strict standards and deliver prototypes quickly. The table below highlights some key challenges you may face with medical devices and devices:
Description | |
|---|---|
Reliability and Stability | Ensuring exceptional reliability and stability to guarantee patient safety over prolonged use. |
Regulatory Compliance | Meeting stringent standards such as ISO 13485, FDA, and CE to comply with medical regulations. |
Design Complexity | Managing advanced routing, connectivity, and thermal considerations in sophisticated designs. |
You can use a step-by-step approach to evaluate certifications, turnaround, and support for your next prototype.
Key Takeaways
Choose a PCBA partner with strong medical certifications like ISO 13485 and FDA compliance to ensure safety and regulatory approval.
Prioritize quick turnaround times by simplifying designs and working closely with your manufacturer to speed up prototyping.
Demand thorough quality control with advanced inspections like AOI and X-ray to guarantee reliable and safe medical device prototypes.
Seek engineering support early to optimize your design for manufacturing and avoid costly delays or redesigns.
Look for transparent pricing and positive customer reviews to find a trustworthy partner who delivers quality on time and within budget.
Certifications & Compliance

ISO, FDA, and Medical Standards
When you choose a partner for medical pcb assembly, you must prioritize certifications that guarantee safety, reliability, and regulatory acceptance. Medical devices face some of the strictest requirements in the electronics industry. You need to ensure your prototypes meet all mandatory standards before moving forward.
Here are the essential certifications and standards you should look for:
ISO 13485:2016: This quality management system standard is specific to medical devices. It ensures your manufacturer follows strict processes for risk management, documentation, and regulatory compliance throughout the product lifecycle.
FDA Regulations (21 CFR Part 820): If you plan to market in the United States, you must comply with FDA requirements. These include design controls, traceability, and post-market surveillance for every medical pcb assembly.
IEC 60601-1: This standard covers electrical safety and performance for medical electrical equipment. It addresses requirements like dielectric strength, EMI control, and thermal management.
UL 60601-1: Many healthcare providers require this certification. It focuses on fire hazards, flammability, and overall safety.
IPC Standards (IPC-A-600, IPC-A-610, IPC-A-6012): These industry standards define the acceptability and performance criteria for PCB fabrication and assembly. They ensure your high-quality prototypes meet strict reliability benchmarks.
Tip: Early integration of these standards in your design and prototyping process helps you avoid costly revisions and ensures a smoother path to regulatory approval.
You can compare the focus and requirements of these standards in the table below:
Standard Body | Standard(s) | Focus and Requirements |
|---|---|---|
IPC | IPC-A-600, IPC-A-610, IPC-A-6012 | Manufacturing quality, reliability, and performance criteria for PCBs and assemblies |
ISO | ISO 9001:2015, ISO 13485:2016 | Quality management, risk management, and documentation for medical devices |
FDA | CFR 820, device classification, premarket submissions | Legal compliance, design control, traceability, and post-market surveillance |
RoHS | Lead-free, hazardous material restrictions | Environmental safety for global markets |
IEC | IEC 60601 | Electrical safety and performance for medical equipment |
By ensuring your local medical pcb manufacturer meets these requirements, you protect your project from regulatory setbacks and guarantee patient safety.
LTPCBA’s Quality Assurance
LTPCBA stands out as a leader in compliance and quality for medical pcb assembly. You benefit from their commitment to international standards and advanced quality management systems.
LTPCBA holds ISO 9001 and ISO 13485 certifications, which are critical for medical device safety and reliability.
The company maintains a robust Quality Management System that covers planning, control, inspection, and problem resolution.
You can rely on advanced inspection methods, including Automated Optical Inspection (AOI), X-ray inspection, and In-Circuit Testing (ICT), to ensure every board meets strict industry standards.
LTPCBA enforces strict material traceability, so you always know the origin and quality of every component in your high-quality prototypes.
The company’s continuous improvement practices, such as statistical process control and detailed defect tracking, help maintain a 99.5% pass rate.
LTPCBA provides detailed quality reports and supports ongoing compliance with evolving regulations, including RoHS amendments.
When you work with LTPCBA, you gain a partner who understands the demands of medical devices and delivers consistent results. Their dedication to compliance and quality assurance gives you confidence in every stage of your project.
Quick Turnaround & Speed

Factors Affecting Lead Time
You need a quickturn pcb solution when developing medical devices. Fast prototyping helps you accelerate time-to-market and stay ahead of competitors. Several factors influence turnaround time for a quick turn pcba prototype medical device. Design complexity plays a major role. Boards with more layers, tighter trace widths, and dense component placement require longer manufacturing cycles. Material selection also impacts turnaround time. Standard FR-4 materials support rapid turnaround time, while specialty materials like polyimide may extend lead times. Manufacturing processes such as drilling, plating, and surface finishing add to production time. Testing and quality assurance, including burn-in and 100% X-ray inspection for medical devices, can increase turnaround time. You can reduce delays by simplifying your design, choosing standard materials, and maintaining clear communication with your manufacturer.
Tip: Early collaboration with your quickturn pcb provider helps you optimize design and material choices, reducing turnaround time and avoiding costly rework.
Here is a table showing typical turnaround times for medical device prototypes based on PCB complexity:
PCB Complexity | Typical Turnaround Time | Notes on Medical Device Sector Relevance |
|---|---|---|
Standard double-sided (FR4) | Next-day (24 hours) | Suitable for simpler medical device PCBA prototypes |
4 to 8 layers | 2 to 4 days | Common complexity range for medical device PCBA |
10 or more layers | 5 days | Reflects higher complexity often required in medical PCBA |
LTPCBA’s Quick Response
LTPCBA delivers quickturn pcb services that meet the demands of medical devices. You receive quotes for your quick turn pcba prototype medical device within 2-3 working days. The company maintains a rapid turnaround time, completing simple 2-layer quickturn pcb orders in just 1 day and more complex prototypes in 3 to 14 days. LTPCBA’s smart machines and automated systems support fast job completion. You benefit from 24/7 multi-channel support, dedicated account managers, and early design reviews. These services help you avoid bottlenecks and keep your project on schedule. LTPCBA’s high on-time delivery rate—over 97%—ensures your quickturn pcb prototype arrives when you need it.
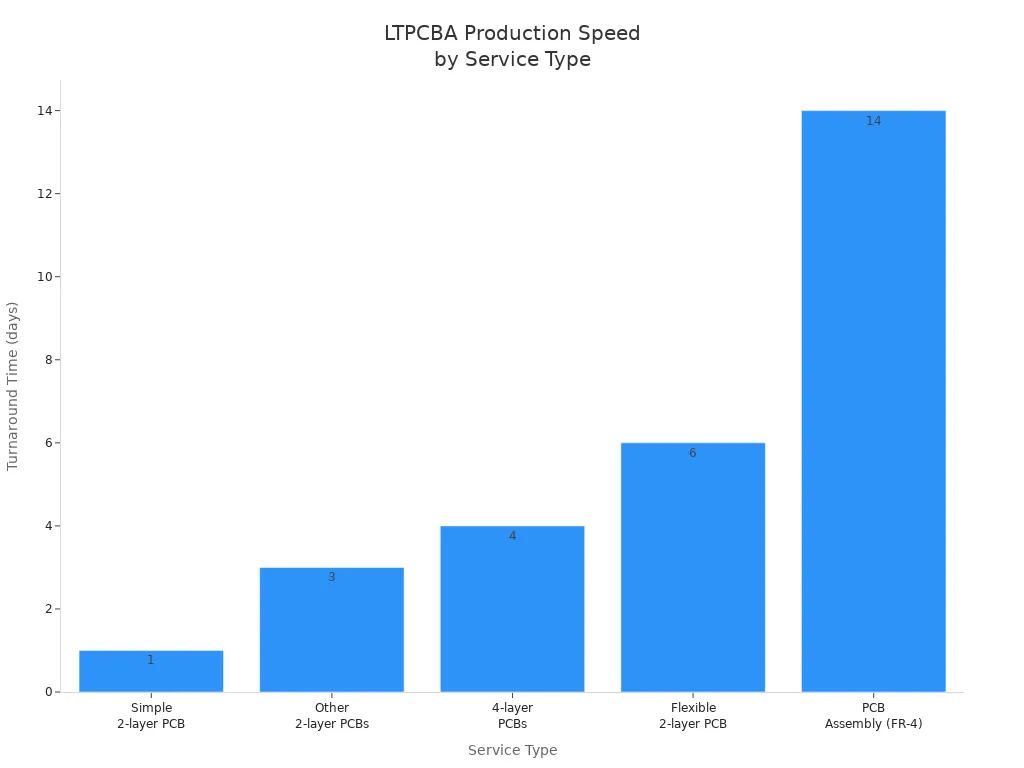
LTPCBA’s rapid turnaround time and quick response help you shorten development cycles and accelerate time-to-market for medical devices.
quick turn pcba prototype medical device
You need a quick turn pcba prototype medical device partner who understands the urgency of medical device innovation. LTPCBA’s quickturn pcb capabilities allow you to iterate designs quickly and respond to regulatory feedback without delay. Fast turnaround time supports agile development and enables you to deliver safe, reliable devices to market faster. When you choose LTPCBA for your quick turn pcba prototype medical device, you gain a competitive edge and ensure your prototypes meet strict quality standards.
Quality Control & Traceability
Inspection & Testing
You must demand rigorous inspection and testing for every medical pcb assembly. Medical devices require high reliability, so you need advanced methods like automated optical inspection (AOI) and X-ray imaging. AOI uses high-resolution cameras and smart image processing to catch surface defects such as solder bridges, misaligned components, and insufficient solder. This technology reduces inspection time and flags issues early, preventing costly rework. X-ray systems complement AOI by detecting hidden flaws in solder joints and internal layers. You should expect your quickturn pcb provider to combine these methods with functional and environmental tests. These protocols push each quickturn pcb prototype to its limits, ensuring durability and safety.
AOI and X-ray inspection help you meet strict standards like IPC-A-600 and IPC-A-610, which are critical for medical pcb assembly. You gain confidence that your prototypes will perform reliably in real-world medical environments.
Focus Area | Purpose | Importance for Medical Devices | |
|---|---|---|---|
Before component mounting | Bare board characteristics | Detect early defects | Ensures design and process compliance |
After component mounting | Solder joints, component layout | Ensure assembly quality | Critical for reliability and safety |
Documentation & Traceability
You need complete documentation and traceable manufacturing processes for every quickturn pcb prototype. Regulatory bodies like the FDA and EMA require you to record every design decision, component source, and test result. This documentation supports regulatory submissions and helps you troubleshoot or update your device. Traceability means you can track every part and process step from prototyping to delivery. Barcode scanning and database management systems link serial numbers to work orders and supplier data. These practices protect patient safety and support rapid response to recalls or quality issues.
Proper documentation and traceability are essential for passing audits and maintaining compliance with ISO 13485. You demonstrate accountability and ensure your medical pcb assembly meets global standards.
quickturn pcb prototype
When you choose LTPCBA for your quickturn pcb prototype, you benefit from advanced inspection, robust documentation, and strict traceability. LTPCBA maintains cleanrooms, ESD-safe workstations, and controlled environments to protect sensitive medical electronics. The company holds ISO 13485 and ISO 9001 certifications, conducts regular audits, and uses documented CAPA procedures. You receive a turn-key pcb order with synchronized quality management and design updates. LTPCBA’s traceable manufacturing processes ensure every quickturn pcb meets the highest safety and reliability standards for medical devices.
Engineering Support & Expertise
DFM & Technical Consultation
You need more than just fast assembly for medical device prototyping. Engineering expertise and design for manufacturability (DFM) support play a critical role in your success. When you work with a medical electronics contract manufacturer, you gain access to multidisciplinary engineering skills. These include electrical, mechanical, and software engineering, as well as deep knowledge of medical-grade standards like IEC 60601.
A strong DFM process helps you avoid common pitfalls such as poor trace routing, incompatible component placement, and assembly bottlenecks. Early technical consultation allows you to identify and fix issues like thermal stress, non-standard components, and test coverage gaps. This approach reduces costly redesigns and accelerates your time to market. You benefit from cross-functional collaboration, which aligns your design with real-world manufacturing needs and ensures your prototype meets strict regulatory requirements.
Tip: Early DFM reviews help you optimize component selection, validate your BOM, and confirm testability, making your medical device development smoother and more reliable.
LTPCBA’s Customer Support
LTPCBA stands out as a medical electronics contract manufacturer by offering dedicated project guidance and 24/7 support. You receive hands-on assistance from experienced engineers who understand the unique challenges of medical device prototyping. LTPCBA’s support team uses multiple channels—email, chat, phone, and dedicated account managers—to keep your project on track at every stage.
Unlike many providers who limit support to business hours, LTPCBA remains available around the clock. This continuous availability helps you resolve issues quickly and avoid delays. The company’s strong quality assurance, ISO and IPC certifications, and cleanroom environments further enhance your confidence in their r&d and production capabilities.
medical electronics contract manufacturer
Choosing the right medical electronics contract manufacturer gives you a competitive edge. LTPCBA combines advanced engineering support, robust DFM processes, and responsive customer service. You benefit from their experience with complex medical projects and their commitment to high-quality prototype delivery. As a trusted medical electronics contract manufacturer, LTPCBA helps you navigate regulatory requirements, optimize your designs, and bring innovative medical devices to market faster.
Cost, Value & Reputation
Transparent Pricing
You need to understand the true cost of quickturn PCBA prototyping for medical devices. Transparent pricing models help you manage your budget and avoid hidden fees. When you request a quote, ask for a detailed breakdown that includes materials, production, testing, and any additional services. This approach gives you full visibility into manufacturing expenses and helps you plan for multiple prototype versions or spares. Transparent pricing also supports long-term financial planning and reduces the risk of cost overruns.
Competitive pricing should be balanced with quality and turnaround time.
The cheapest option may not deliver high-quality prototypes or reliable support.
Manufacturers with clear pricing structures make it easier for you to control costs and accelerate your project.
One-stop PCBA services simplify project management and reduce indirect costs.
You should compare formal quotes from several manufacturers and choose partners who offer quickturn services at reasonable costs. Collaborate closely to implement design changes cost-effectively and ensure your devices meet strict standards.
Prototype Type | Typical Price Range (Single Unit) | Notes |
|---|---|---|
Simple Double-Layer PCB | Standard specs, fast turnaround time | |
Complex Multilayer PCB | $500 - $1,500 | Advanced features, longer turnaround time |
Flex PCB Prototype | $300 - $800 | Flexible circuits, moderate turnaround time |
Aluminum PCB Prototype | $400 - $1,200+ | Specialized material, variable turnaround time |
IoT Device PCB Prototype | $200 - $400 | Compact, quickturn, for connected devices |
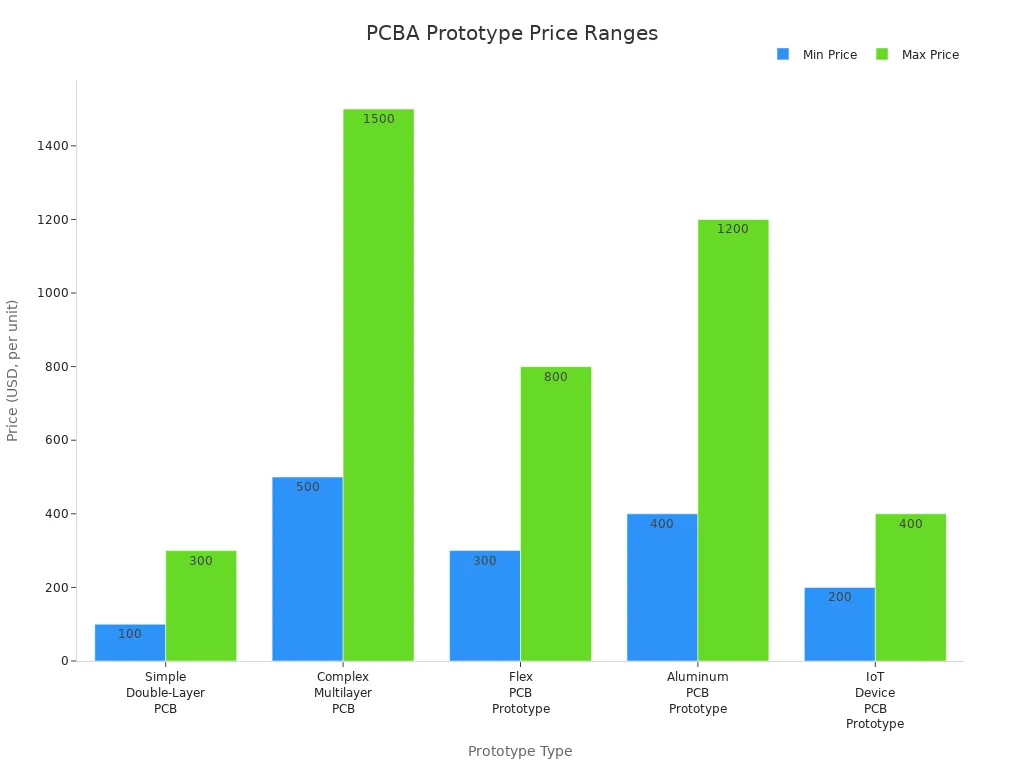
Transparent pricing and predictable turnaround time help you budget effectively and bring your devices to market faster.
Customer Reviews
Customer feedback gives you valuable insight into the reliability and quality of quickturn PCBA services. LTPCBA receives high ratings for its 99.5% first-pass yield, which exceeds industry averages. You benefit from a 98% on-time delivery rate and responsive communication, supported by 24/7 technical support and a real-time project tracking portal. Customers praise LTPCBA’s advanced inspection methods, including AOI and X-ray testing, which ensure high-quality prototypes for medical devices. The company’s ability to handle complex assemblies and deliver consistent results builds trust and confidence.
LTPCBA holds ISO 9001, IPC-A-610, IATF 16949, and UL certifications.
Customers highlight reliable quickturn service and strong technical expertise.
Real-time tracking and responsive support improve your experience and project outcomes.
LTPCBA’s Industry Experience
You want a partner with proven experience in quickturn PCBA prototyping for medical devices. LTPCBA stands out with its achievements and commitment to quality. The company maintains a 99.5% pass rate and invests in state-of-the-art equipment, including automated pick-and-place machines and flexible SMT lines. LTPCBA’s cleanroom environments and strict quality control processes ensure your high-quality prototypes meet regulatory requirements. The team’s expertise in handling complex devices and scaling from prototyping to production supports your innovation and growth.
LTPCBA’s industry partnerships and certifications demonstrate reliability.
The company’s quickturn capabilities and fast turnaround time help you stay ahead in the competitive medical device market.
You benefit from comprehensive support, advanced technology, and a reputation for excellence.
You can choose the right quick turn PCBA prototyping service for medical devices by focusing on these essential criteria:
Prioritize fast turnaround and reliable quality control.
Seek strong engineering support and transparent pricing.
Review customer feedback and industry reputation.
Successful companies use these steps to plan, iterate, and manage risk. You can follow this checklist to accelerate development and ensure compliance. Consider LTPCBA for your next medical device prototype to achieve rapid, reliable results.
FAQ
What certifications should you look for in a medical device PCBA partner?
You should check for ISO 13485, ISO 9001, and IPC-A-610 certifications. These show that the manufacturer meets strict quality and safety standards for medical devices.
How fast can you get a quick turn PCBA prototype for a medical device?
You can receive simple prototypes in as little as 24 hours. More complex boards may take 3 to 14 days. LTPCBA provides rapid quotes and fast production to meet urgent project needs.
How does LTPCBA ensure quality and traceability?
LTPCBA uses automated optical inspection, X-ray testing, and strict documentation. You can track every component and process step. This ensures your prototype meets regulatory requirements and supports full traceability.
What support does LTPCBA offer during prototyping?
You get 24/7 technical support, direct access to engineers, and real-time project updates. LTPCBA helps you solve problems quickly and keeps your project on schedule.
See Also
Choosing The Best Turnkey PCBA Manufacturer For Electronics
Understanding PCBA Processing Needs For Medical Devices
Obtaining Fast Turnaround PCB Prototypes Within The USA
